Our Mission.
Curadigm is dedicated to transforming leading-edge therapeutics and nanotechnologies into real-world clinical solutions for patients. Our approach utilizes innovative concepts on the frontiers of physics, chemistry and biology to redefine how therapeutics interact with the body. Through our novel body priming technology, which is administered prior to the therapeutic to prevent rapid drug clearance, we believe we can revolutionize treatment success by allowing the drug to get to the right place at the right time.
Our deep expertise in nanomedicines and their biological interactions allows us to use engineered design to rethink how therapeutics are cleared from and function within the body.
Importantly, our technology does not require modifying the therapeutic in any way, which could compromise its efficacy. Our approach allows us to redefine systemic bioavailability, enabling therapeutics hindered by poor bioavailability and low accumulation in target tissues, to become successful, driving the development of treatments to benefit patients that need them most.
Our Company.
Curadigm is an early-stage company focused on shifting the paradigm of therapeutic clearance, bioavailability, and efficacy to improve treatment outcomes for millions of patients. Curadigm was spun-off from Nanobiotix, a late-stage clinical company, in 2019 and maintains their commitment to cutting-edge therapeutic approaches.
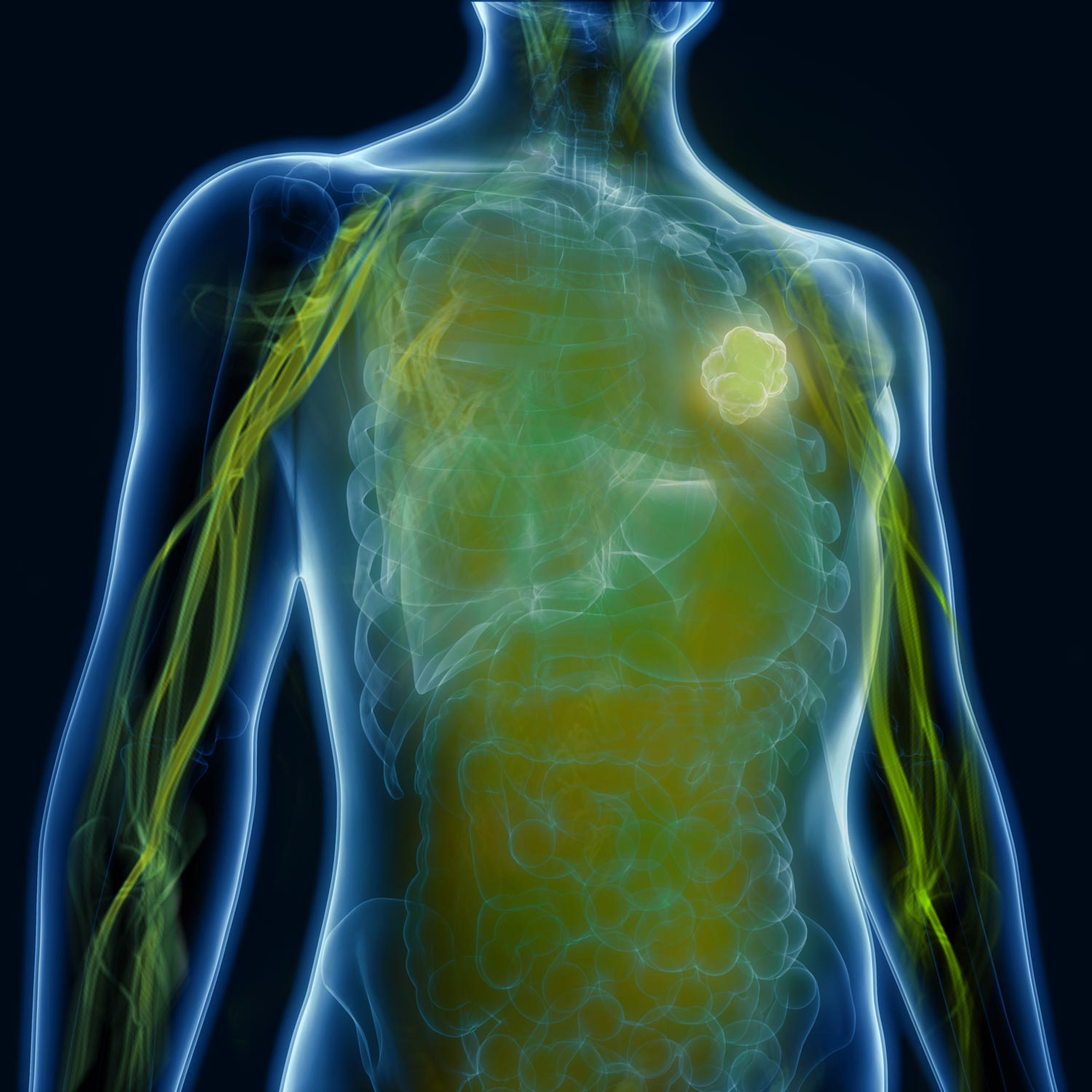
Curadigm has developed an industry-first nanotechnology designed to increase bioavailability and efficacy, while reducing off-target effects. Our product is administered intravenously just before the drug and “primes” the body to receive treatment by reducing rapid therapeutic clearance and hepatic toxicity. Curadigm’s priming technology is flexible and can be adapted to work with multiple therapeutic classes, including nanomedicines, nucleic acid therapeutics, small molecules, and gene editing technologies.
Curadigm has ongoing programs and collaborations across many therapeutic classes, disease indications, and target tissues to improve bioavailability and efficacy. The company is also actively pursuing a therapeutic “rescue” pipeline to redefine the therapeutic profile of compounds that have failed in late stage clinical trials due to lack of efficacy or hepatic toxicity.
Our Technology.
The Curadigm Concept
Solution driven Science
Curadigm’s technology was developed to address a critical challenge facing intravenously administered therapeutics: How can you efficiently deliver a drug to your tissue of interest without rapid clearance? To address this, we developed technology that reduces unwanted hepatic clearance, resulting in a temporary increase in blood bioavailability so the therapeutic they can reach its target sites. This leads to an enhanced treatment effect, all without modifying the therapeutic in any way.
Shifting the therapeutic paradigm to elevate therapeutic outcomes.
Current Approach
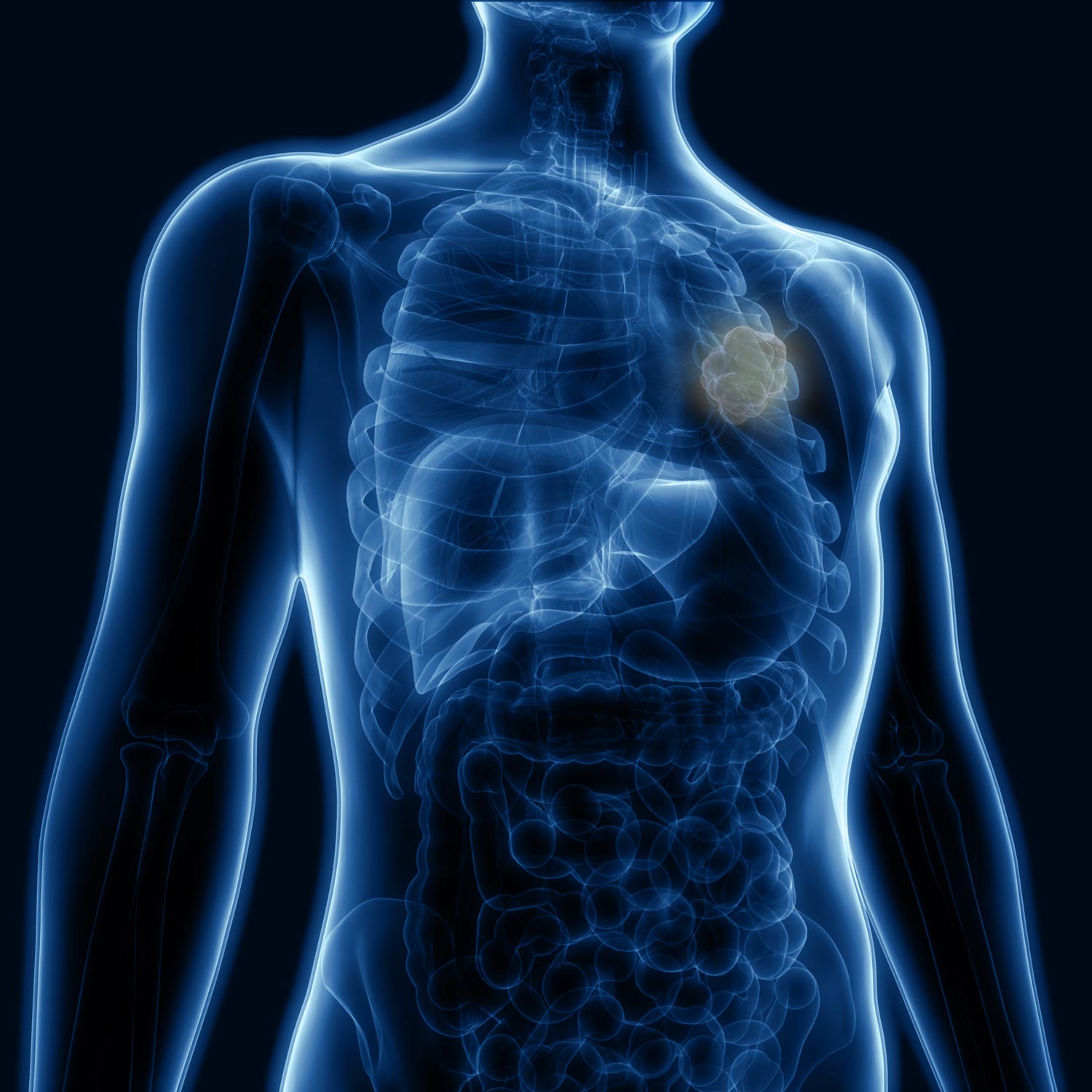
Treat Alone
The Curadigm Solution
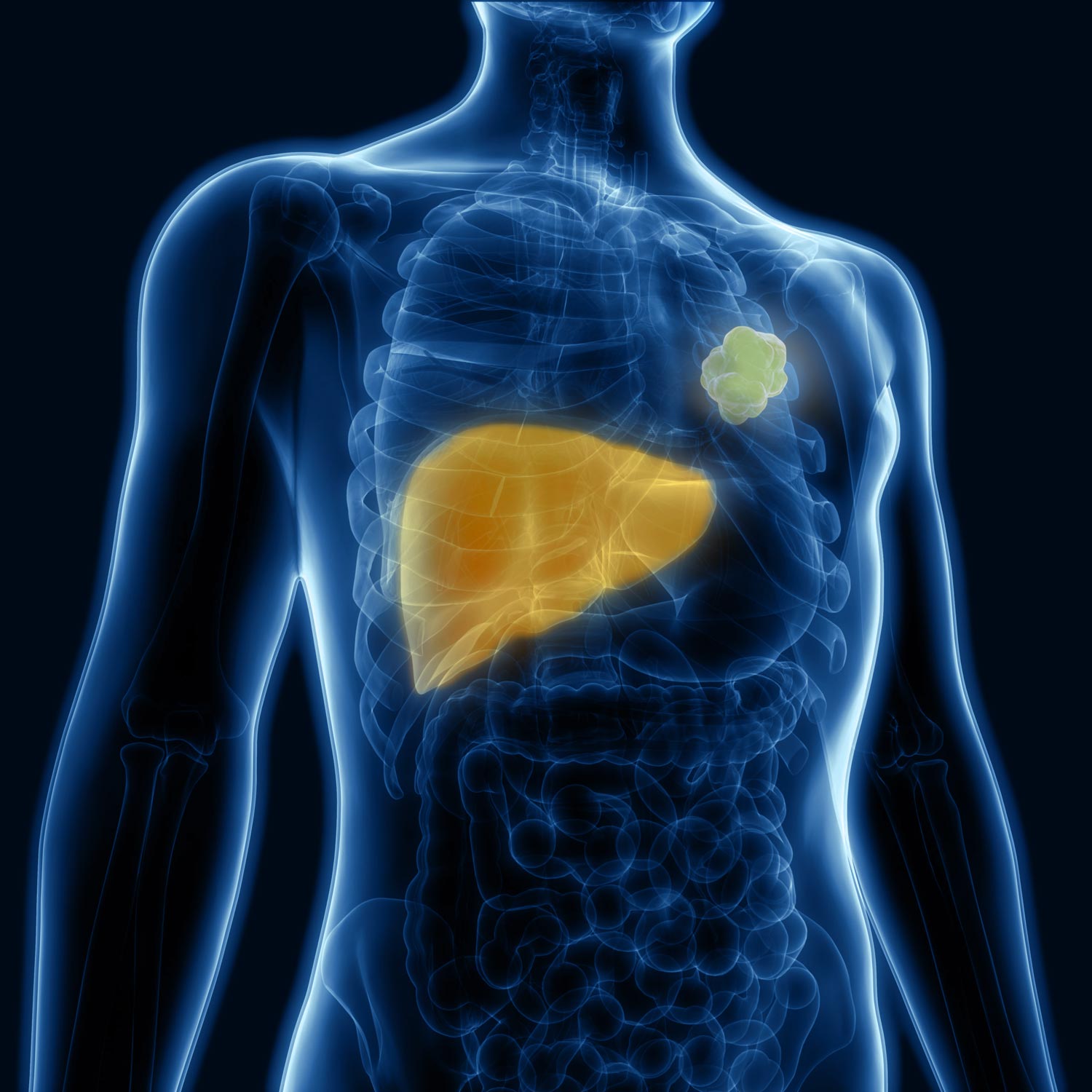
01. Prime the Body to Receive Treatment
The Curadigm Advantages.
The Nanoprimer is designed to impact only the cells involved in therapeutic clearance while leaving other liver functions undisturbed. This is accomplished by our precisely engineered lipid-based nanoparticle with specific physio-chemical properties that allow it to occupy Kupffer cells and liver sinusoidal endothelial cells (LSEC) that are involved in the clearance of most intravenous therapeutics. The Nanoprimer features simultaneously prevent it from accessing other liver-cell types, such as hepatocytes, that perform critical functions such as metabolization and detoxification.
The Nanoprimer effect is based solely on these properties and it does not contain any drugs, biologics or antibody targeting.
01. Prime
Nanoprimer specifically & transiently occupies liver clearance pathways.

02. Treat
Therapeutic undergoes reduced clearance & increased bioavailability.
Increases blood bioavailability for only a short time-frame
Uncouples therapeutic blood bioavailability & delivery, allowing optimized drug function
Nanoprimer does not contain drugs or biologics to occupy the liver that may impact multiple pathways or elicit immune response
Nanopriming with Nucleic-acid therapeutics.
The Nanoprimer platform is particularly impactful in combination with nucleic-acid based therapeutics. These drugs, which include RNA-based therapies such as mRNAs and siRNAs, as well as DNA-based therapies, which include CRISPR-Cas9 and DNA encoding technologies, generally require encapsulation in nanoparticles for intravenous administration.
This leads to a high level of rapid clearance by the liver, a challenge that has limited the ability of these types of therapeutics to target non-hepatic tissues. The Nanoprimer can be used in combination with these classes of therapeutics to prevent rapid Kupffer-based clearance, increase blood bioavailability, and improve accumulation in target tissues.